The backbone of affordable health care in the United States is generic medications. About 90 percent of all prescriptions filled in the U.S. are for generic medications (U.S. Food and Drug Administration, 2000). In addition to being less costly than brand-name drugs, generic medications offer patients safe and reliable treatment options. Providers must know about generic drugs to prescribe them effectively; therefore, understanding generics will help providers plan and implement comprehensive, effective treatment programs for their patients and assist in lowering the cost of providing health care services.
This article examines how generic drugs are approved, the strict quality standards they must meet, their advantages and drawbacks, and key points healthcare workers should keep in mind.
What Are Generic Drugs?
Generic drugs work the same way as brand-name drugs and are made to have the same therapeutic effect. They need to have the exact:
- Main ingredient
- Strength of dose
- Form (like a pill, liquid, or shot)
- The way they are taken
- Medical purpose
Even though generic drugs might not look the same, they must provide the same health benefits as their brand-name counterparts.
Manufacturers can start producing generic drugs once the original drug’s patents and rights expire. When this happens, multiple companies can make generic versions, boosting competition and bringing down prices.
How the FDA Approves Generic Drugs
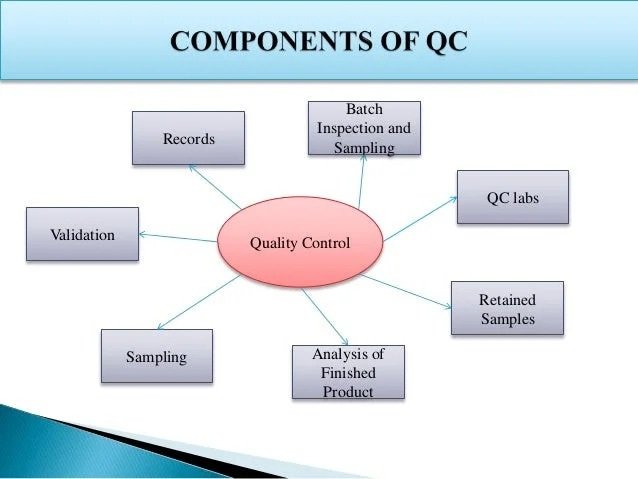
The FDA reviews these drugs to ensure they comply with strict safety and effectiveness standards.
Abbreviated New Drug Application (ANDA)
Companies file an ANDA to prove their generic product works the same way as the original brand-name drug.
This involves several steps:
Bioequivalence Testing
Bioequivalence checks make sure that:
- The generic medicine enters the bloodstream at a similar speed
- The active ingredient absorbed is almost the same amount
These tests confirm the treatment’s effectiveness stays the same as the brand-name version.
Manufacturing Inspections
The FDA visits production sites to ensure they follow Current Good Manufacturing Practices (CGMP). These rules include:
- Keeping production areas clean
- Ensuring proper labeling
- Checking ingredients
- Verifying quality through testing
Stability and Storage Requirements
Generic drugs need to stay safe and work well during their entire shelf life when stored properly.
The FDA approves them after they meet all these standards.
Why Are Generic Drugs Important in Healthcare?
Reduced Treatment Expenses
Generic drugs cost much less than brand-name ones. In many cases, they are 80 percent or more cheaper. This lower cost helps:
- People who pay out of pocket
- Insurance companies
- Programs funded by the government
Cheaper drugs mean patients can stick to their treatments because the cost is less of a burden.
Wider Access to Treatment
Generic drugs help healthcare providers treat more people in areas where medical resources are lacking. This access helps control long-term illnesses such as:
- Diabetes
- High blood pressure
- Heart disease
- Asthma
Proven Effectiveness
The FDA requires that generic drugs be as effective as brand-name drugs. Research shows no noticeable difference in how they affect health results.
Market Stability
Making generics involves multiple companies, reducing the risk of supply shortages and keeping essential medicines available to patients.
Answering Questions About Generic Drugs
Even though generics have strong evidence supporting their use, questions still arise sometimes.
Quality Worries
Both generic and brand-name drugs approved by the FDA follow the same safety and production standards.
Doubts About Effectiveness
Tests for bioequivalence confirm that generics provide similar therapeutic results.
Side Effects
The side effects are similar, though the inactive ingredients might differ slightly. These minor differences rarely change how the drug works.
Doctors can ease concerns by sharing information about FDA rules and research results.
Times to Be Careful
Switching between different manufacturers might need extra attention with some medicines.
Narrow Therapeutic Index (NTI) Drugs
These medicines have a narrow range between effectiveness and risk. Examples include:
- Blood thinners
- Some thyroid medicines
- Drugs for preventing seizures
In these situations, doctors suggest sticking with the same manufacturer and regularly monitoring the patient’s response.
How Pharmaceutical Distribution Affects Access to Generic Drugs
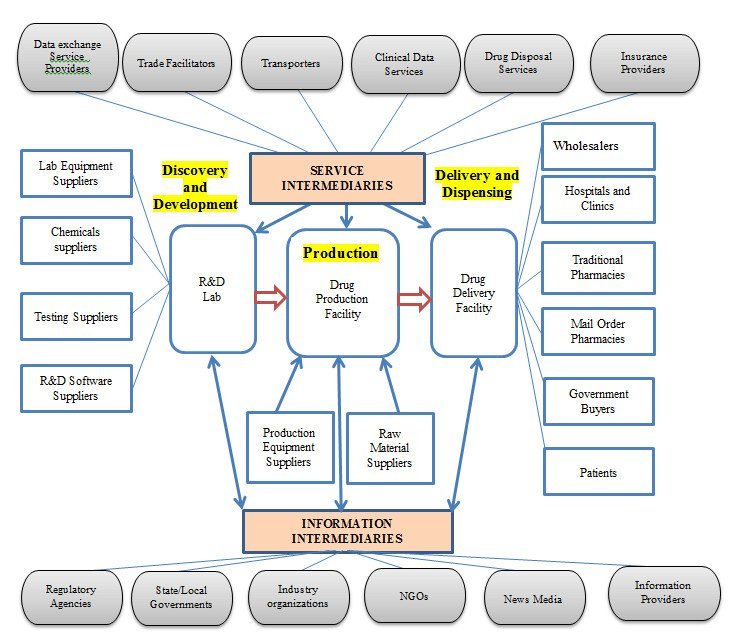
After approval, generic drugs undergo a controlled system before they are available to patients.
A dependable pharmaceutical distribution company has a vital role in:
- Keeping medicines stored
- Following all regulatory rules
- Making sure medications arrive
- Handling inventory well
The collaboration between healthcare facilities and wholesale pharmaceutical suppliers enables both to place larger orders for generic medicines. This improved pricing and assured, timely, reliable supply of essential medications.
Effective distribution networks also help prevent drug shortages and maintain the quality of medicine.
Guidelines for Prescribing Generic Drugs
Healthcare workers can improve the use of generic drugs by using these methods:
Inform Patients
Make sure to explain:
- How the FDA approves medications
- The safety measures in place
- The cost advantages
Simple explanations can ease worries and support better patient compliance.
Look Over Insurance Options
Insurance promotes generic medications by offering lower copays and broader coverage to consumers.
Check Treatment Results
Healthcare providers should monitor their efficacy and/or adverse effects when switching between suppliers/manufacturers.
Consider What Patients Want
Some people trust brand-name drugs more. Providers can ease concerns by sharing evidence-based facts.
Generics in Various Healthcare Areas
Hospitals
Hospitals depend heavily on generics to keep pharmacy costs lower while delivering quality healthcare.
Primary Care Clinics
Doctors in clinics often prescribe generics to help manage chronic conditions over time.
Nursing Homes and Elder Care
Cheaper options help keep care steady and affordable for older adults.
Keeping Drugs Safe After Approval
The FDA keeps checking drug safety even after it approves them through:
- Systems to report adverse events
- Routine facility checks
- Recalling products if needed
Healthcare workers help by:
- Notifying about side effects
- Keeping up with FDA news
- Teaching patients proper usage
These checks and efforts boost confidence in generic drugs.
Worldwide Oversight of Manufacturing
Companies outside the U.S. make many generic medicines. The FDA inspects to maintain quality.
They check:
- How drugs are made
- Cleanliness of facilities
- Tests for quality
- Records of production
If a facility does not meet standards, it cannot provide drugs in the U.S.
Expanding Role of Biosimilars
Biosimilars are close versions of complex biological drugs. These are like generic drugs but are used to provide cheaper treatments.
People now use biosimilars more often in areas like:
- Cancer treatments
- Managing autoimmune diseases
- Specialized therapies
The FDA ensures biosimilars meet strict safety and effectiveness standards.
How Generic Drugs Affect Healthcare
Health systems that encourage doctors to prescribe generic drugs notice:
- Reduced drug costs
- Better patient use of medications
- More patients are getting the treatment they need
Community clinics often use generics to treat large numbers of patients with fewer resources. Hospitals save money by choosing generics, which allows them to spend more on staff training, better technology, and expanded patient care.
Building Trust through FDA Regulations
The FDA shares public details about:
- Approved generic drugs
- Inspection results
- Safety notices
Healthcare professionals can use this information to stay up to date and trust the medications they prescribe.
What Lies Ahead
As patents on brand-name drugs run out, more generic drugs will hit the market. This ongoing shift will:
- Lower medical expenses
- Improve availability for patients
- Build more sustainable healthcare
Better oversight and improved supply chains will continue to boost quality standards.
Final Thoughts
FDA-approved generics provide doctors and patients with reliable, effective, and budget-friendly treatment options. Strict approval rules, regular safety checks, and high manufacturing standards ensure generics deliver the same benefits as brand-name options for much less money.
By understanding how generics are managed and supplied, healthcare workers can prescribe them with confidence, inform patients, and help improve health outcomes while keeping costs under control.
Medical Disclaimer
This information is intended to inform and does not replace professional medical guidance. Providers should use their clinical expertise and follow official prescribing guidelines when prescribing.
References
- U.S. Food and Drug Administration (FDA). Generic Drug Facts
- U.S. Food and Drug Administration (FDA). Abbreviated New Drug Application (ANDA)
- National Institutes of Health (NIH). Generic and Brand-Name Drugs
- Journal of the American Medical Association (JAMA). Outcomes After Switching to Generic Drugs
- U.S. Government Accountability Office (GAO). Generic Drug Oversight

